|
Shown in Figure 2 is a typical contamination vs. time plot.
As per IPC-TM-650 Method 2.3.25 (ROSE Method), ionic residues are expressed
as equivalents of sodium chloride (NaCl) in micrograms per unit surface area.
The sample passes the test if the conductivity (or resistivity) is less than
or equal to the target conductivity (or resistivity) value, and fails otherwise.
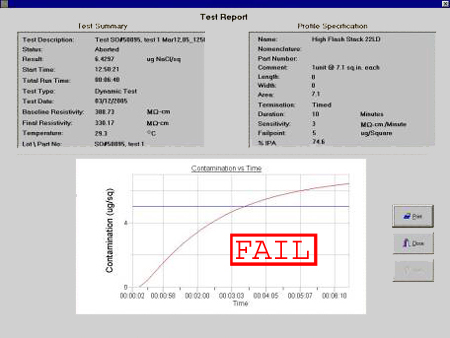
Figure 2. Contamination vs. time graph.
Applicable Specifications & Standards:
|