Ionic Contamination at the Component Level
Introduction
Standards exist for ionic cleanliness and contamination testing at the electronic
board assembly level, but currently none have been established for the component
level.
Ionic contaminants typically are flux residues or harmful materials that are
picked up or left behind during the process. They are generally water-soluble organic
or inorganic acids or salts.
Why test for Ionic Contamination?
Ionic contamination can degrade the reliability of the electronic components
and assemblies as it [1]:
- contributes to current leakage between the circuitry
- promotes dendrite growth
- increases the risk of corrosion
- weakens the bond strength of the solder joints
Shown in Figure 1 are examples of the aforementioned concerns.



Figure.1: (a) Silver dendrites growing on a flexible circuit between silver conducting
tracks (b) Corrosion of copper circuitry on PCB (b) Burnt PCB resulting from arcing,
initially caused by dendrites. [2]
Types of Tests
Ionic Conductivity
Test and Ion Chromatography Test are the two most popular tests used in the industry
to detect ionic contamination.
1) Ionic Conductivity
Test
Ionic conductivity tests are performed by removing the ionic residues from the component
and measuring the conductivity (or resistivity) of the extract solution. Generally
the extract solution used for the test is 75/25% (isopropyl alcohol/de-ionized water).
Ionic residues are usually expressed as equivalence of sodium chloride in micrograms
per square centimeter of the sample (mg NaCl Eq./cm2).
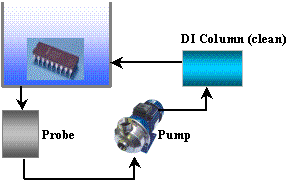
Figure 2. Schematic of the Dynamic Test
The dynamic method, as shown in Figure 2, is the most commonly used in the industry.
In this method, a purified alcohol/water mixture is circulated into and out of the
test tank chamber containing the sample being tested. The mixture exiting the test
tank is passed through a conductivity cell, which measures the conductivity continuously.
This method does not differentiate between ionic species.
2) Ion chromatography
Figure 3. Schematic of Ion Chromatography.
Ion Chromatography (as illustrated in Figure 3) is a technique for separating
the components, or solutes, of a mixture on the basis of the relative amounts of
each solute distributed between a moving fluid stream, called the mobile phase,
and a contiguous stationary phase. The liquid mobile phase permeates through a stationary
phase and elutes the solutes into a flow-through detector. This method can differentiate
between different ionic species. Results are expressed as micrograms of each species
per square centimeter of the sample (mg ion/cm2).
Ionic Cleanliness testing at the component level is strongly recommended for
high reliability Military and Aerospace applications.
For more information:
Visit: www.sixsigmaservices.com
E-mail: sales@solderquik.com
Call: 408-956-0100
For over 13 years Six Sigma has performed ionic cleanliness testing
on components for commercial, military, and aerospace customers. During this time
Six Sigma has processed over 20 million components for high reliability applications.
Due to the lack of an industry standard, Six Sigma has written its own specifications
for testing microelectronic components.
References:
[1] N.C. Lee , Reflow Soldering Processes and Troubleshooting: SMT, BGA, CSP
and Flip Chip Technologies, (Butterworth-Heinemann Publishing, Woburn, Massachusetts,
2002, pp.86-87.
[2] Contamination and moisture effects on printed circuit board reliability.
ERA Technology [Online]. Available at
http://www.era.co.uk/news/rfa_feature_05a.asp
(accessed July 31, 2005)
Six Sigma: Subscribe
|